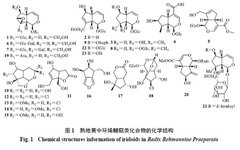
 Rehmannia glutinosa (熟地黄) is a plant of the Scrophulariaceae family. It is processed from fresh or dried tubers. It is sweet and slightly warm in nature, and it enters the liver and kidney meridians. It has the effects of nourishing blood, enriching yin, and filling the marrow, and can be used to treat conditions such as blood deficiency, palpitations, irregular menstruation, excessive bleeding, liver and kidney yin deficiency, soreness of the lower back and knees, bone steaming heat, night sweats, premature ejaculation, internal heat leading to thirst, dizziness, tinnitus, and premature graying of hair.[1]The processing methods of Rehmannia glutinosa were first recorded in the “Discussion on Processing” by Lei Xiu during the Northern and Southern Dynasties: “Harvest fresh Rehmannia, remove the white skin, steam it in a porcelain pot with willow wood, let it cool, mix with wine and steam again, then dry it, avoiding contact with copper and iron utensils.” The processing methods have evolved from the initial two steams and two dries to nine steams and nine dries, and from a single auxiliary material (wine) to multiple auxiliary materials (wine, Amomum, Poria, etc.). Different processing methods also directly affect the changes in the chemical composition of Rehmannia and the differences in its pharmacological effects.[2]Rehmannia glutinosa is one of the most representative herbs for the “nourishing yin” effect in traditional Chinese medicine, also known as the “ultimate yin herb.” When combined with related formulas, it can be applied to various clinical diseases caused by liver and kidney yin deficiency, including diabetes-related diseases (type 2 diabetes[3], diabetic nephropathy[4], and insulin resistance[5]), neurological diseases (Alzheimer’s disease[6], Parkinson’s disease[7], and vascular dementia[8]), osteoarthritis-related diseases (postmenopausal osteoporosis[9] and rheumatoid arthritis[10]), and gynecological diseases (various symptoms before and after menopause[11], kidney yin deficiency type amenorrhea[12], insufficient ovarian reserve[13], premature ovarian failure secondary amenorrhea[14], kidney yin deficiency type perimenopausal syndrome[15], and menopausal syndrome[16]). The pharmacological basis and efficacy mechanisms of Rehmannia glutinosa in different disease states and combinations remain unclear and require further in-depth exploration. The pharmacological basis refers to the active chemical substances contained in traditional Chinese medicine or formulas that can express their traditional clinical efficacy, including both direct acting substances and those that act after metabolism. Effective and active components are both included in the scope of pharmacological basis research.[17]Pharmacological basis research is fundamental and a prerequisite for elucidating the mechanisms of action of traditional Chinese medicine, developing new drugs, and broad applications, and it is a scientific problem that urgently needs to be solved by modern science and technology. This article aims to review the research progress on the chemical composition, pharmacological effects of active components, and the pharmacological basis under the conditions of formula combinations of Rehmannia glutinosa, in order to provide references for related research.1 Chemical CompositionStudies have shown that Rehmannia glutinosa mainly contains iridoids, phenolic compounds, and phenylethanoid compounds, as well as carbohydrates, nucleosides, alkaloids, phenolic acids, isoflavonoids, and other compounds.1.1 IridoidsThe iridoid components are the most abundant and highest in content in Rehmannia glutinosa, with the basic skeleton primarily based on Rehmannia glycosides, and the sugar moieties predominantly being glucose. Identified iridoid compounds in Rehmannia glutinosa[18-20] include Rehmannioside A (1), Rehmannioside B (2), Rehmannioside C (3), 8-epiloganic acid (4), Ginsenoside (5), Rehmannin A (6), Rehmannin B (7), Rehmannin C (8), Rehmannin D (9), Rehmannin A (10), Rehmannin C (11), Rehmannin D (12),焦地黄素A (13), 焦地黄素B (14), 焦地黄素C (15), 焦地黄呋喃 (16), 焦地黄内酯 (17), Chlorinated Rehmannin (18), 焦地黄苷A (19), 焦地黄苷B (20), 6-O-E-feruloyl-β-D-glucopyranoside (21), Miltefosine (22), Monomiltefosine (23), etc. The chemical structures are shown in Figure 1.
Rehmannia glutinosa (熟地黄) is a plant of the Scrophulariaceae family. It is processed from fresh or dried tubers. It is sweet and slightly warm in nature, and it enters the liver and kidney meridians. It has the effects of nourishing blood, enriching yin, and filling the marrow, and can be used to treat conditions such as blood deficiency, palpitations, irregular menstruation, excessive bleeding, liver and kidney yin deficiency, soreness of the lower back and knees, bone steaming heat, night sweats, premature ejaculation, internal heat leading to thirst, dizziness, tinnitus, and premature graying of hair.[1]The processing methods of Rehmannia glutinosa were first recorded in the “Discussion on Processing” by Lei Xiu during the Northern and Southern Dynasties: “Harvest fresh Rehmannia, remove the white skin, steam it in a porcelain pot with willow wood, let it cool, mix with wine and steam again, then dry it, avoiding contact with copper and iron utensils.” The processing methods have evolved from the initial two steams and two dries to nine steams and nine dries, and from a single auxiliary material (wine) to multiple auxiliary materials (wine, Amomum, Poria, etc.). Different processing methods also directly affect the changes in the chemical composition of Rehmannia and the differences in its pharmacological effects.[2]Rehmannia glutinosa is one of the most representative herbs for the “nourishing yin” effect in traditional Chinese medicine, also known as the “ultimate yin herb.” When combined with related formulas, it can be applied to various clinical diseases caused by liver and kidney yin deficiency, including diabetes-related diseases (type 2 diabetes[3], diabetic nephropathy[4], and insulin resistance[5]), neurological diseases (Alzheimer’s disease[6], Parkinson’s disease[7], and vascular dementia[8]), osteoarthritis-related diseases (postmenopausal osteoporosis[9] and rheumatoid arthritis[10]), and gynecological diseases (various symptoms before and after menopause[11], kidney yin deficiency type amenorrhea[12], insufficient ovarian reserve[13], premature ovarian failure secondary amenorrhea[14], kidney yin deficiency type perimenopausal syndrome[15], and menopausal syndrome[16]). The pharmacological basis and efficacy mechanisms of Rehmannia glutinosa in different disease states and combinations remain unclear and require further in-depth exploration. The pharmacological basis refers to the active chemical substances contained in traditional Chinese medicine or formulas that can express their traditional clinical efficacy, including both direct acting substances and those that act after metabolism. Effective and active components are both included in the scope of pharmacological basis research.[17]Pharmacological basis research is fundamental and a prerequisite for elucidating the mechanisms of action of traditional Chinese medicine, developing new drugs, and broad applications, and it is a scientific problem that urgently needs to be solved by modern science and technology. This article aims to review the research progress on the chemical composition, pharmacological effects of active components, and the pharmacological basis under the conditions of formula combinations of Rehmannia glutinosa, in order to provide references for related research.1 Chemical CompositionStudies have shown that Rehmannia glutinosa mainly contains iridoids, phenolic compounds, and phenylethanoid compounds, as well as carbohydrates, nucleosides, alkaloids, phenolic acids, isoflavonoids, and other compounds.1.1 IridoidsThe iridoid components are the most abundant and highest in content in Rehmannia glutinosa, with the basic skeleton primarily based on Rehmannia glycosides, and the sugar moieties predominantly being glucose. Identified iridoid compounds in Rehmannia glutinosa[18-20] include Rehmannioside A (1), Rehmannioside B (2), Rehmannioside C (3), 8-epiloganic acid (4), Ginsenoside (5), Rehmannin A (6), Rehmannin B (7), Rehmannin C (8), Rehmannin D (9), Rehmannin A (10), Rehmannin C (11), Rehmannin D (12),焦地黄素A (13), 焦地黄素B (14), 焦地黄素C (15), 焦地黄呋喃 (16), 焦地黄内酯 (17), Chlorinated Rehmannin (18), 焦地黄苷A (19), 焦地黄苷B (20), 6-O-E-feruloyl-β-D-glucopyranoside (21), Miltefosine (22), Monomiltefosine (23), etc. The chemical structures are shown in Figure 1.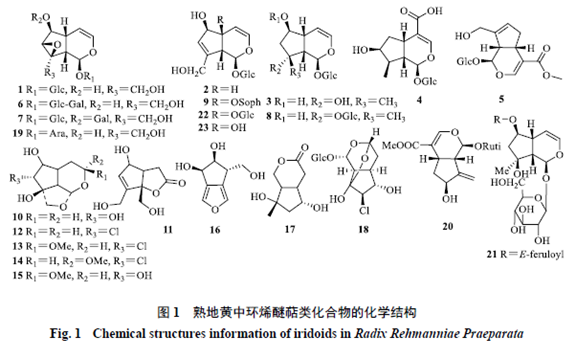 1.2 Phenolic CompoundsPhenolic compounds identified in Rehmannia glutinosa are primarily monoterpenes and sesquiterpenes, characterized by dimethyl substitution at C1 and a double bond between C5 and C6, with C5 being able to glycosylate with sugars, occasionally polymerizing with iridoids. Identified phenolic compounds in Rehmannia glutinosa[18,20-21] include Rehmannia phenolic glycoside A (24), Rehmannia phenolic glycoside B (25), Wild Cudrania acid (26), 5-Hydroxy Wild Cudrania acid (27), Rehmannia bitter glycoside (28), Dihydroxy-β-phenolic (29), Trihydroxy-β-phenolic (30), Rehmannia bitter aglycone (31), Frehmaglutin A (32), sec-hydroxyaeginetic acid-2-O-β-D-glucopyranoside (33), etc. The chemical structures are shown in Figure 2.
1.2 Phenolic CompoundsPhenolic compounds identified in Rehmannia glutinosa are primarily monoterpenes and sesquiterpenes, characterized by dimethyl substitution at C1 and a double bond between C5 and C6, with C5 being able to glycosylate with sugars, occasionally polymerizing with iridoids. Identified phenolic compounds in Rehmannia glutinosa[18,20-21] include Rehmannia phenolic glycoside A (24), Rehmannia phenolic glycoside B (25), Wild Cudrania acid (26), 5-Hydroxy Wild Cudrania acid (27), Rehmannia bitter glycoside (28), Dihydroxy-β-phenolic (29), Trihydroxy-β-phenolic (30), Rehmannia bitter aglycone (31), Frehmaglutin A (32), sec-hydroxyaeginetic acid-2-O-β-D-glucopyranoside (33), etc. The chemical structures are shown in Figure 2.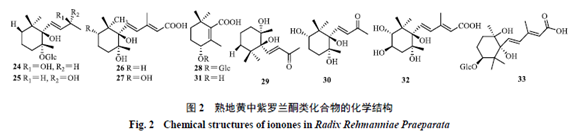 1.3 Phenylethanoid CompoundsMost phenylethanoid compounds in Rehmannia glutinosa exist in glycoside form, characterized by β-glucose as the connecting body, with phenylethanoid and phenylacetyl as substituents, connected to different positions of the sugar by glycosidic bonds. Identified phenylethanoid compounds in Rehmannia glutinosa[18,20,22-23] include Echinacoside (34), Hairy Flower Sugar Glycoside (35),焦地黄苯乙醇苷A1 (36), 焦地黄苯乙醇苷B1 (37), Isohairy Flower Sugar Glycoside (38), Cistanche glycoside F (39), Rehmannia glycoside (40), Iso-Rehmannia glycoside (41), Digitalis leaf glycoside C (42), p-Hydroxyphenylethanol (43), etc. The chemical structures are shown in Figure 3.
1.3 Phenylethanoid CompoundsMost phenylethanoid compounds in Rehmannia glutinosa exist in glycoside form, characterized by β-glucose as the connecting body, with phenylethanoid and phenylacetyl as substituents, connected to different positions of the sugar by glycosidic bonds. Identified phenylethanoid compounds in Rehmannia glutinosa[18,20,22-23] include Echinacoside (34), Hairy Flower Sugar Glycoside (35),焦地黄苯乙醇苷A1 (36), 焦地黄苯乙醇苷B1 (37), Isohairy Flower Sugar Glycoside (38), Cistanche glycoside F (39), Rehmannia glycoside (40), Iso-Rehmannia glycoside (41), Digitalis leaf glycoside C (42), p-Hydroxyphenylethanol (43), etc. The chemical structures are shown in Figure 3.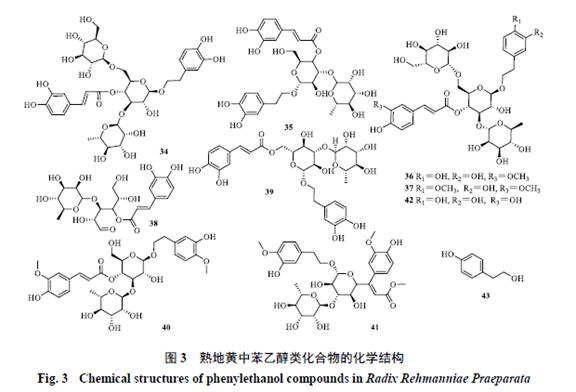 1.4 Carbohydrates and Nucleosidesand NucleosidesIdentified carbohydrate components in Rehmannia glutinosa[24] include galactose, glucose, sucrose, rhamnose, maltose, cottonseed sugar, mannotriose, fructose, hairy flower sugar, etc. Nucleoside components[20,25] include adenine, uracil, inosine, cytidine, hypoxanthine, guanosine, uridine, adenosine, etc. The identified carbohydrate and nucleoside components in Rehmannia glutinosa are shown in Table 1.
1.4 Carbohydrates and Nucleosidesand NucleosidesIdentified carbohydrate components in Rehmannia glutinosa[24] include galactose, glucose, sucrose, rhamnose, maltose, cottonseed sugar, mannotriose, fructose, hairy flower sugar, etc. Nucleoside components[20,25] include adenine, uracil, inosine, cytidine, hypoxanthine, guanosine, uridine, adenosine, etc. The identified carbohydrate and nucleoside components in Rehmannia glutinosa are shown in Table 1.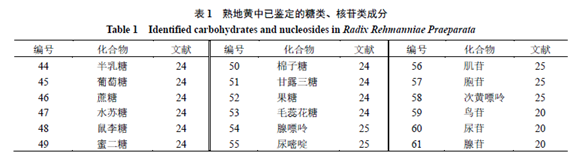 1.5 Alkaloids, Phenolic Acids, and IsoflavonoidsOther components identified in Rehmannia glutinosa include alkaloids, phenolic acids, and isoflavonoids, respectively (62~67, 68~78, 79~82), and other components (83~92). The component information is detailed in Table 2, and the representative component chemical structures are shown in Figure 4.
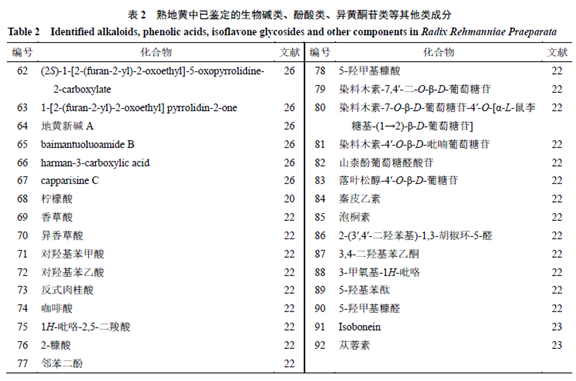
1.5 Alkaloids, Phenolic Acids, and IsoflavonoidsOther components identified in Rehmannia glutinosa include alkaloids, phenolic acids, and isoflavonoids, respectively (62~67, 68~78, 79~82), and other components (83~92). The component information is detailed in Table 2, and the representative component chemical structures are shown in Figure 4.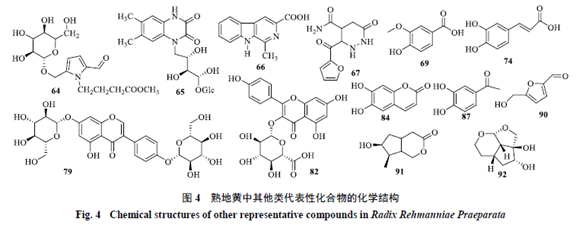
 2 Pharmacological Effects2.1 IridoidsStudies have shown that Rehmannin has cognitive improvement, neuroprotection, and antidepressant-like effects on the nervous system, as well as blood sugar lowering, anti-tumor, and cardiovascular protection effects[27]; Rehmannin can improve non-alcoholic fatty liver based on the endoplasmic reticulum stress-mediated protein kinase RNA-like endoplasmic reticulum kinase-eukaryotic initiation factor 2α antibody signaling pathway[28]; Rehmannin protects against liver damage in diabetic rats, possibly through the peroxisome proliferator-activated receptor γ (PPARγ)/nuclear factor-κB (NF-κB) signaling pathway to reduce inflammation[29], and may lower blood sugar levels in diabetic rats by activating PPARγ coactivator-1α signaling pathway[30]. Studies have shown that Peach Leaf Coral Glycoside has a wide range of pharmacological effects, including antioxidant, anti-inflammatory, anti-fibrotic, and anti-tumor effects, with significant protective effects on various tissues and organs, regulating multiple signaling pathways such as nuclear factor erythroid 2-related factor 2 (Nrf2), NF-κB, adenosine monophosphate activated protein kinase (AMPK), nitric oxide, and C-Jun N-terminal kinase[31]; recent studies have shown that Peach Leaf Coral Glycoside can inhibit high glucose-induced renal tubular epithelial cell inflammatory damage by upregulating micro RNA-374 (miR-374) expression[32]. Rehmannioside can promote the biogenesis of lysosomes and the generation of autophagic lysosomes by inhibiting mammalian target of rapamycin (mTOR) phosphorylation, promoting lipid autophagy, and reducing liver lipid accumulation, thereby improving hepatic steatosis[33]; Rehmannioside effectively inhibits the proliferation of human esophageal cancer TE-1, Eca-109, and KYSE-150 cells, causing varying degrees of apoptosis and blocking the cancer cell proliferation cycle at the G2/M phase[34]. Studies have shown that Ginsenoside has a wide range of pharmacological activities, regulating mouse glucose and lipid metabolism[35], anti-diabetic effects[36], anti-cholestatic liver injury[37], anti-pulmonary fibrosis[38], improving skeletal muscle function[39], and neuroprotection[40]; recent studies have shown that Ginsenoside protects against diabetic nephropathy in mice through the pre-dynamic protein 2/pre-dynamic protein receptor 1 signaling pathway[41]; Ginsenoside inhibits oxidative stress and apoptosis in a Parkinson’s model through the AMPK/mTOR pathway[42]; Ginsenoside inhibits the growth of diffuse large B-cell lymphoma through the long-chain non-coding RNA HLA complex P5/miR-27b-3p/stromal epithelial transition factor axis[43]; Ginsenoside can regulate serotonin metabolism pathways to improve oxidative stress in the brain, exerting neuroprotective effects[44]. Studies have shown that Rehmannin A has a nourishing yin effect and enhances both humoral and cellular immunity[45], significantly improving cyclophosphamide-induced leukopenia in mice[46]. Rehmannin D may inhibit the activation of microglia by adjusting the release of inflammatory factors, suppressing the transformation of microglia from M2 to M1, thereby improving neuronal inflammation[47]. In the pharmacochemical study of fresh Rehmannia serum, it was found that Rehmannin[48], Rehmannioside[48], Rehmannin A[49], and Rehmannin D[48] all enter the blood in their original forms, and in vitro pharmacological activity studies suggest that these chemical components may be the pharmacological basis of Rehmannia glutinosa. It has also been confirmed that the content of Rehmannin and Rehmannioside decreases during the processing of Rehmannia, while the content of Rehmannin D remains relatively stable[50]. This suggests that the differences in pharmacological effects of fresh, raw, and cooked Rehmannia may be related to changes in the content of active components before and after processing.2.2 Phenolic CompoundsStudies have shown that Rehmannia bitter aglycone can alleviate insulin resistance in polycystic ovary syndrome model rats and restore ovarian morphology, possibly related to the regulation of phosphatidylinositol 3-kinase/extracellular signal-regulated kinase pathway[51]. Studies have shown that Rehmannia bitter aglycone has estrogen-like activity and mediates through estrogen receptor α and G protein-coupled receptor 30[52]. Research by Zheng Xiaoke et al.[53] found that Rehmannia bitter glycoside and Rehmannia bitter aglycone have immunosuppressive activity. These studies suggest that Rehmannia bitter glycoside and Rehmannia bitter aglycone may be the pharmacological basis of Rehmannia glutinosa.2.3 Phenylethanoid CompoundsStudies have shown that Echinacoside may improve glutamate-induced damage to rat adrenal chromaffin PC-12 cells by reducing intracellular Ca2+ accumulation, inhibiting the expression of ionotropic glutamate receptor 1 antibody protein, and exerting antioxidant effects[54]; Echinacoside may reduce apoptosis levels by inhibiting mitochondrial apoptosis signaling pathways to improve cortisone-induced PC-12 cell damage[55]. These studies suggest that Echinacoside may be one of the pharmacological bases for the antidepressant effects of Rehmannia; Echinacoside can also improve the ultrastructure of midbrain neurons in Parkinson’s model rats, which may be related to alleviating mitochondrial dysfunction in neurons[56]; Echinacoside can reduce liver ischemia-reperfusion injury in rats by activating the Nrf2 signaling pathway[57]; Echinacoside can alleviate acute kidney injury in severely burned rats by inhibiting oxidative stress damage, inflammatory response, and apoptosis, with molecular mechanisms related to activating silent information regulator 3 (SIRT3) signaling pathway[58]; Echinacoside is a potential therapeutic agent for sepsis-related liver injury and metabolic disorders, possibly acting through mediating the activation of phosphorylated signal transducer and activator of transcription 3 (p-STAT3) and phosphorylated protein kinase B (p-Akt) in liver tissues[59]; Echinacoside’s protective effects on diabetic myocardium may be related to negative feedback regulation of transforming growth factor-β1/Smads signaling pathway[60].Studies have shown that Hairy Flower Sugar Glycoside has significant antidepressant effects, possibly related to increased monoamine neurotransmitters, reduced pro-inflammatory factors, and restoring neurotransmitter balance by increasing γ-aminobutyric acid, mainly acting through interactions with neuroactive ligands-receptors, GABAergic synapses, synaptic vesicle cycling, and cyclic adenosine monophosphate signaling pathways[61]; Hairy Flower Sugar Glycoside can protect against hypoxia and re-perfusion-induced neuronal damage through the mitochondrial cysteine aspartate protease-3/poly(ADP-ribose) polymerase apoptosis pathway[62]; Hairy Flower Sugar Glycoside can improve renal lesions in diabetic nephropathy mice, possibly related to reducing high mobility group protein 1 and NF-κB expression[63]; Hairy Flower Sugar Glycoside may inhibit excessive immune stress in the body by downregulating the expression of zinc finger transcription factors, thereby inhibiting the epithelial-mesenchymal transition of renal tubular epithelial cells, delaying renal function deterioration[64]; Hairy Flower Sugar Glycoside can treat chronic heart failure by improving cardiac hemodynamics and reducing sympathetic nervous system excitability[65]; Hairy Flower Sugar Glycoside can inhibit STAT3 phosphorylation by upregulating the expression of tyrosine phosphatase 1, producing anti-glioblastoma effects[66], and downregulating CD44 expression to inhibit epithelial-mesenchymal transition of glioma cells[67]; Hairy Flower Sugar Glycoside has a moderate inhibitory effect on cytochrome P450 3A4 enzyme in rat liver microsomes, with affinity comparable to positive inhibitors (α-naphthoflavone, thiazolidinedione, sulfanilamide, etc.)[68]. Studies have shown that Isohairy Flower Sugar Glycoside can inhibit the growth of human ovarian cancer OVCAR-3 cells both in vitro and in vivo, suggesting it may serve as a potential anti-cancer agent[69]. Both Hairy Flower Sugar Glycoside and Isohairy Flower Sugar Glycoside can improve cognitive dysfunction induced by β-amyloid 1-42 by blocking amyloid oligomerization, counteracting the cytotoxicity of amyloid and reversing central nervous system function[70]. Cistanche glycoside F can significantly increase the survival rate of human liver HL-7702 cells, showing notable in vitro hepatoprotective activity[23]. These studies suggest that Echinacoside, Hairy Flower Sugar Glycoside, Isohairy Flower Sugar Glycoside, and Cistanche glycoside F may be the pharmacological basis of Rehmannia glutinosa.2.4 CarbohydratesStudies have shown that through gas chromatography and Fourier transform infrared spectroscopy, the polysaccharides of Rehmannia glutinosa are composed of rhamnose, arabinose, mannose, glucose, and galactose in a molar ratio of 1.00:1.26:0.73:16.45:30.40, with an average relative molecular weight of 63,500. Rehmannia polysaccharides can effectively improve hyperglycemia, hyperlipidemia, vascular inflammation, and oxidative stress in streptozotocin-induced diabetic mice, making it a potential treatment option for type 1 diabetes[71]; Rehmannia polysaccharides may regulate the bone metabolic state in type 2 diabetic rats by activating the Wnt pathway[72]; Rehmannia polysaccharides can improve thyroid function in pregnant mice with hyperthyroidism, alleviating renal damage in offspring, possibly through the Nrf2/heme oxygenase-1/NOD-like receptor thermal protein domain associated protein 3 signaling pathway[73]; Rehmannia polysaccharides can effectively inhibit apoptosis and oxidative damage in rat hippocampal neurons induced by hypoxia-reoxygenation, possibly related to inhibiting the circular RNA 0010729/miR-326 pathway[74]; Rehmannia polysaccharides can reduce vascular calcification induced by vitamin D3 and nicotine in rats, possibly by inhibiting vascular cell apoptosis, thus preventing osteoblastic transformation and calcification of vascular cells[75]; Rehmannia polysaccharides can improve learning and memory impairment induced by scopolamine in mice, with effects related to regulating cholinergic nervous system function, antioxidant stress, and inhibiting inflammatory responses[76]; exercise training combined with Rehmannia polysaccharides can repair neurological function in rats after cerebral ischemia-reperfusion, improving learning and memory abilities, possibly through reducing oxidative stress responses, inhibiting NF-κB pathway activation, and slowing the progression of inflammation[77].2.5 Alkaloids, Phenolic Acids, and IsoflavonoidsStudies have shown that the alkaloids Rehmannine A, Baimantuoluoamide B, and Capparisine C in Rehmannia glutinosa have protective effects against lipopolysaccharide-induced damage in rat renal tubular epithelial NRK-52e cells[26]. Vanillic acid can alleviate palmitic acid-induced fatty degeneration in human liver cancer HepG2 cells by inducing autophagy, indicating its significance in finding potential drugs and targets for the prevention and treatment of non-alcoholic fatty liver disease. Studies have shown that caffeic acid can protect against doxorubicin-induced myocardial injury by reducing oxidative stress, lowering mitochondrial membrane potential, and increasing reactive oxygen species levels in cardiomyocytes H9c2, and by regulating NF-κB signaling pathway[79]. Quercetin-3-O-glucuronide may be the molecular entity exerting hypoglycemic effects, acting directly on the Akt PH region to activate Akt, exerting hypoglycemic effects through the Akt-glycogen synthase kinase-3β pathway[80]. In evaluating the responses of estrogen target cells to a series of isoflavonoid glycosides and aglycones from Genista halacsyi Heldr., it was found that compared to aglycones, daidzein-7-O-β-D-pyranoglucoside and daidzein-7,4′-di-O-β-D-pyranoglucoside have higher estrogen-like and anti-inflammatory activities, suggesting that formulations rich in daidzein-7-O-β-D-pyranoglucoside can be used as alternatives to low-dose estrogen drugs for treating menopausal symptoms[81].2.6 Other Active ComponentsStudies have shown that Qinpi Ethyl Ester has therapeutic effects on lung cancer, gastric cancer, liver cancer, pancreatic cancer, intestinal cancer, prostate cancer, breast cancer, melanoma, brain glioma, multiple myeloma, leukemia, etc., inhibiting cell proliferation, inducing apoptosis, blocking the cell cycle, inhibiting tumor metastasis, suppressing angiogenesis, enhancing immunity, and sensitizing and reducing toxicity[82]. Recent studies have shown that Qinpi Ethyl Ester may inhibit the proliferation, migration, and invasion of human cervical cancer SiHa cells by downregulating FLVCR1-AS1 gene levels[83]; Qinpi Ethyl Ester can inhibit the growth of mouse lung cancer Lewis cell tumors by inducing apoptosis and inhibiting Wnt/β-catenin pathway activation[84]; Qinpi Ethyl Ester has been shown to inhibit the proliferation and stemness of human ovarian cancer SKOV3 cells, while also inducing apoptosis in SKOV3 cells[85]; Qinpi Ethyl Ester can inhibit high glucose-induced renal tubular epithelial cell inflammatory damage by downregulating miR-486-5p expression[86]; Qinpi Ethyl Ester protects human retinal pigment epithelial cell line ARPE-19 from oxidative damage by upregulating intracellular antioxidant enzyme activity or the expression of antioxidant proteins[87].Studies have shown that 3,4-dihydroxyacetophenone has anti-inflammatory and antioxidant effects, and is expected to become an effective drug for treating acute lung injury[88]; 3,4-dihydroxyacetophenone may lower triglyceride levels in liver cells and liver tissues through the AMPK pathway[89]; 3,4-dihydroxyacetophenone effectively inhibits the inflammatory response of lipopolysaccharide-induced macrophages RAW264.7 by inhibiting NF-κB nuclear translocation[90]; 3,4-dihydroxyacetophenone has protective effects on myocardium[91]; 3,4-dihydroxyacetophenone may relax pulmonary arterial smooth muscle cells by opening potassium channels, which may be one of its important mechanisms for lowering pulmonary arterial hypertension[92]; 3,4-dihydroxyacetophenone’s mechanism of inhibiting platelet aggregation may be related to inhibiting thromboxane B2 release in the arachidonic acid metabolic pathway[93].5 Pharmacological Basis Research under Formula CombinationsLiu Shaobo et al.[100] used ultra-performance liquid chromatography-quadrupole-time of flight-mass spectrometry (UPLC-Q-TOF-MS) technology to replicate a kidney yin deficiency model in rats using hydrocortisone, finding that Zhibai Dihuang Wan can effectively alleviate kidney yin deficiency, and the active components belonging to Rehmannia glutinosa in the serum of the treated rats mainly include Hairy Flower Sugar, Rhamnose, Maltose, and Berberine sulfate glucuronide. Liu Kaixin et al.[101] used UPLC-Q-TOF-MS technology to replicate a bone marrow suppression model in rats using recombinant human granulocyte-stimulating factor, finding that Shengyu Decoction significantly increased the body weight, hemoglobin, red blood cell count, and platelet count in bone marrow suppression rats; under effective conditions, a total of 26 prototype components and 20 metabolites were characterized, among which the blood components belonging to Rehmannia glutinosa included Rehmannin, Rehmannin metabolites, Hairy Flower Sugar glycoside metabolites, and Rehmannin D metabolites. Duan et al.[102] used UPLC-Q-TOF-MS technology to study the effects of Taohong Siwu Decoction on SD rats, identifying a total of 39 active components and 90 metabolites in the blood and brain tissues of the rats, among which the prototype components derived from Rehmannia glutinosa included焦地黄苯乙醇苷B1, Astragalin, and Rehmannin D. Wang et al.[103] used UPLC-Q-TOF-MS technology to study the effects of Zuogui Wan on SD rats, detecting prototype components from Rehmannia glutinosa in rat serum samples, including β-D-ribofuranuronic acid methyl ester triacetate, 5-hydroxymethyl-2-furfural glucuronide, and dihydro-5-hydroxymethyl-2-furfural glucuronide, with metabolites being 3-hydroxy-2,6,6-trimethyl-1-cyclohexene-1-carboxylic acid. Yang Huajie et al.[104] used UPLC-Q-TOF-MS technology to find that in SD rats treated with Liuwei Dihuang Wan, a total of 32 migrating components were detected in rat serum, among which the chemical components derived from Rehmannia glutinosa included 8-epiloganic acid, Gardenia glycoside, acetyl Rehmannin, Hairy Flower Sugar glycoside, Rehmannin, Iso-Rehmannin, oleanolic acid, mannitol, dibutyl phthalate, and protocatechuic acid. Compounds absorbed into the human/animal body in the form of prototype components or metabolites are likely to be the pharmacological basis for regulating diseases, so under effective conditions, compounds detected in animals through high-resolution separation and identification techniques are more worthy of attention and further pharmacological activity research.4 Conclusion and OutlookThis study reviews the research progress on the chemical composition, pharmacological effects of active components, and the pharmacological basis under the conditions of formula combinations of Rehmannia glutinosa, finding that Rehmannia glutinosa mainly contains various types of compounds such as iridoids, phenolic compounds, and phenylethanoid compounds. The pharmacologically active components in Rehmannia glutinosa, including Rehmannin D, Rehmannin, Peach Leaf Coral Glycoside, Rehmannioside, Ginsenoside, Rehmannia bitter glycoside, Echinacoside, Hairy Flower Sugar Glycoside, Rehmannia polysaccharides, Qinpi Ethyl Ester, 3,4-dihydroxyacetophenone, and 5-hydroxymethylfurfural, may be the chemical basis for its therapeutic effects. The pharmacological effects are mainly reflected in blood sugar lowering, anti-tumor, improving liver fat, antidepressant, improving cognitive impairment, and having estrogen-like activity. The uncertainty of effective components and unclear mechanisms of action have always been a bottleneck restricting the modernization of traditional Chinese medicine. In recent years, the continuous development of serum pharmacochemistry and high-resolution separation and identification technologies has accelerated research related to the pharmacological basis of traditional Chinese medicine. Serum pharmacochemistry has achieved a leap from in vitro to in vivo research on active components of traditional Chinese medicine, allowing for the characterization of compounds that exert effects through absorption into the serum and further pharmacokinetic and mechanism analysis, deepening researchers’ understanding of effective components of traditional Chinese medicine. Further practice and exploration in related research are needed. Traditional Chinese medicine has characteristics of “holism, multi-targeting, and synergy,” and research on the pharmacological basis of traditional Chinese medicine must align with the fundamental theories of traditional Chinese medicine, rather than focusing solely on single active components. The multi-level data integration concept of systems biology is closely related to the “holistic view” of traditional Chinese medicine, encompassing genomics, transcriptomics, proteomics, metabolomics, and network pharmacology, achieving new breakthroughs in the evaluation of efficacy and mechanisms of traditional Chinese medicine formulas. Therefore, further developing systems biology research methods may help advance research on the pharmacological basis of traditional Chinese medicine, promoting traditional Chinese medicine to the world stage and better serving the health of all humanity.Conflict of InterestAll authors declare no conflict of interest.References (omitted) Source: Ge Nan, Yan Guangli, Sun Hui, Wang Xijun. Research Progress on the Pharmacological Basis of Rehmannia Glutinosa [J]. Chinese Herbal Medicine, 2023, 54(1):292-302.
2 Pharmacological Effects2.1 IridoidsStudies have shown that Rehmannin has cognitive improvement, neuroprotection, and antidepressant-like effects on the nervous system, as well as blood sugar lowering, anti-tumor, and cardiovascular protection effects[27]; Rehmannin can improve non-alcoholic fatty liver based on the endoplasmic reticulum stress-mediated protein kinase RNA-like endoplasmic reticulum kinase-eukaryotic initiation factor 2α antibody signaling pathway[28]; Rehmannin protects against liver damage in diabetic rats, possibly through the peroxisome proliferator-activated receptor γ (PPARγ)/nuclear factor-κB (NF-κB) signaling pathway to reduce inflammation[29], and may lower blood sugar levels in diabetic rats by activating PPARγ coactivator-1α signaling pathway[30]. Studies have shown that Peach Leaf Coral Glycoside has a wide range of pharmacological effects, including antioxidant, anti-inflammatory, anti-fibrotic, and anti-tumor effects, with significant protective effects on various tissues and organs, regulating multiple signaling pathways such as nuclear factor erythroid 2-related factor 2 (Nrf2), NF-κB, adenosine monophosphate activated protein kinase (AMPK), nitric oxide, and C-Jun N-terminal kinase[31]; recent studies have shown that Peach Leaf Coral Glycoside can inhibit high glucose-induced renal tubular epithelial cell inflammatory damage by upregulating micro RNA-374 (miR-374) expression[32]. Rehmannioside can promote the biogenesis of lysosomes and the generation of autophagic lysosomes by inhibiting mammalian target of rapamycin (mTOR) phosphorylation, promoting lipid autophagy, and reducing liver lipid accumulation, thereby improving hepatic steatosis[33]; Rehmannioside effectively inhibits the proliferation of human esophageal cancer TE-1, Eca-109, and KYSE-150 cells, causing varying degrees of apoptosis and blocking the cancer cell proliferation cycle at the G2/M phase[34]. Studies have shown that Ginsenoside has a wide range of pharmacological activities, regulating mouse glucose and lipid metabolism[35], anti-diabetic effects[36], anti-cholestatic liver injury[37], anti-pulmonary fibrosis[38], improving skeletal muscle function[39], and neuroprotection[40]; recent studies have shown that Ginsenoside protects against diabetic nephropathy in mice through the pre-dynamic protein 2/pre-dynamic protein receptor 1 signaling pathway[41]; Ginsenoside inhibits oxidative stress and apoptosis in a Parkinson’s model through the AMPK/mTOR pathway[42]; Ginsenoside inhibits the growth of diffuse large B-cell lymphoma through the long-chain non-coding RNA HLA complex P5/miR-27b-3p/stromal epithelial transition factor axis[43]; Ginsenoside can regulate serotonin metabolism pathways to improve oxidative stress in the brain, exerting neuroprotective effects[44]. Studies have shown that Rehmannin A has a nourishing yin effect and enhances both humoral and cellular immunity[45], significantly improving cyclophosphamide-induced leukopenia in mice[46]. Rehmannin D may inhibit the activation of microglia by adjusting the release of inflammatory factors, suppressing the transformation of microglia from M2 to M1, thereby improving neuronal inflammation[47]. In the pharmacochemical study of fresh Rehmannia serum, it was found that Rehmannin[48], Rehmannioside[48], Rehmannin A[49], and Rehmannin D[48] all enter the blood in their original forms, and in vitro pharmacological activity studies suggest that these chemical components may be the pharmacological basis of Rehmannia glutinosa. It has also been confirmed that the content of Rehmannin and Rehmannioside decreases during the processing of Rehmannia, while the content of Rehmannin D remains relatively stable[50]. This suggests that the differences in pharmacological effects of fresh, raw, and cooked Rehmannia may be related to changes in the content of active components before and after processing.2.2 Phenolic CompoundsStudies have shown that Rehmannia bitter aglycone can alleviate insulin resistance in polycystic ovary syndrome model rats and restore ovarian morphology, possibly related to the regulation of phosphatidylinositol 3-kinase/extracellular signal-regulated kinase pathway[51]. Studies have shown that Rehmannia bitter aglycone has estrogen-like activity and mediates through estrogen receptor α and G protein-coupled receptor 30[52]. Research by Zheng Xiaoke et al.[53] found that Rehmannia bitter glycoside and Rehmannia bitter aglycone have immunosuppressive activity. These studies suggest that Rehmannia bitter glycoside and Rehmannia bitter aglycone may be the pharmacological basis of Rehmannia glutinosa.2.3 Phenylethanoid CompoundsStudies have shown that Echinacoside may improve glutamate-induced damage to rat adrenal chromaffin PC-12 cells by reducing intracellular Ca2+ accumulation, inhibiting the expression of ionotropic glutamate receptor 1 antibody protein, and exerting antioxidant effects[54]; Echinacoside may reduce apoptosis levels by inhibiting mitochondrial apoptosis signaling pathways to improve cortisone-induced PC-12 cell damage[55]. These studies suggest that Echinacoside may be one of the pharmacological bases for the antidepressant effects of Rehmannia; Echinacoside can also improve the ultrastructure of midbrain neurons in Parkinson’s model rats, which may be related to alleviating mitochondrial dysfunction in neurons[56]; Echinacoside can reduce liver ischemia-reperfusion injury in rats by activating the Nrf2 signaling pathway[57]; Echinacoside can alleviate acute kidney injury in severely burned rats by inhibiting oxidative stress damage, inflammatory response, and apoptosis, with molecular mechanisms related to activating silent information regulator 3 (SIRT3) signaling pathway[58]; Echinacoside is a potential therapeutic agent for sepsis-related liver injury and metabolic disorders, possibly acting through mediating the activation of phosphorylated signal transducer and activator of transcription 3 (p-STAT3) and phosphorylated protein kinase B (p-Akt) in liver tissues[59]; Echinacoside’s protective effects on diabetic myocardium may be related to negative feedback regulation of transforming growth factor-β1/Smads signaling pathway[60].Studies have shown that Hairy Flower Sugar Glycoside has significant antidepressant effects, possibly related to increased monoamine neurotransmitters, reduced pro-inflammatory factors, and restoring neurotransmitter balance by increasing γ-aminobutyric acid, mainly acting through interactions with neuroactive ligands-receptors, GABAergic synapses, synaptic vesicle cycling, and cyclic adenosine monophosphate signaling pathways[61]; Hairy Flower Sugar Glycoside can protect against hypoxia and re-perfusion-induced neuronal damage through the mitochondrial cysteine aspartate protease-3/poly(ADP-ribose) polymerase apoptosis pathway[62]; Hairy Flower Sugar Glycoside can improve renal lesions in diabetic nephropathy mice, possibly related to reducing high mobility group protein 1 and NF-κB expression[63]; Hairy Flower Sugar Glycoside may inhibit excessive immune stress in the body by downregulating the expression of zinc finger transcription factors, thereby inhibiting the epithelial-mesenchymal transition of renal tubular epithelial cells, delaying renal function deterioration[64]; Hairy Flower Sugar Glycoside can treat chronic heart failure by improving cardiac hemodynamics and reducing sympathetic nervous system excitability[65]; Hairy Flower Sugar Glycoside can inhibit STAT3 phosphorylation by upregulating the expression of tyrosine phosphatase 1, producing anti-glioblastoma effects[66], and downregulating CD44 expression to inhibit epithelial-mesenchymal transition of glioma cells[67]; Hairy Flower Sugar Glycoside has a moderate inhibitory effect on cytochrome P450 3A4 enzyme in rat liver microsomes, with affinity comparable to positive inhibitors (α-naphthoflavone, thiazolidinedione, sulfanilamide, etc.)[68]. Studies have shown that Isohairy Flower Sugar Glycoside can inhibit the growth of human ovarian cancer OVCAR-3 cells both in vitro and in vivo, suggesting it may serve as a potential anti-cancer agent[69]. Both Hairy Flower Sugar Glycoside and Isohairy Flower Sugar Glycoside can improve cognitive dysfunction induced by β-amyloid 1-42 by blocking amyloid oligomerization, counteracting the cytotoxicity of amyloid and reversing central nervous system function[70]. Cistanche glycoside F can significantly increase the survival rate of human liver HL-7702 cells, showing notable in vitro hepatoprotective activity[23]. These studies suggest that Echinacoside, Hairy Flower Sugar Glycoside, Isohairy Flower Sugar Glycoside, and Cistanche glycoside F may be the pharmacological basis of Rehmannia glutinosa.2.4 CarbohydratesStudies have shown that through gas chromatography and Fourier transform infrared spectroscopy, the polysaccharides of Rehmannia glutinosa are composed of rhamnose, arabinose, mannose, glucose, and galactose in a molar ratio of 1.00:1.26:0.73:16.45:30.40, with an average relative molecular weight of 63,500. Rehmannia polysaccharides can effectively improve hyperglycemia, hyperlipidemia, vascular inflammation, and oxidative stress in streptozotocin-induced diabetic mice, making it a potential treatment option for type 1 diabetes[71]; Rehmannia polysaccharides may regulate the bone metabolic state in type 2 diabetic rats by activating the Wnt pathway[72]; Rehmannia polysaccharides can improve thyroid function in pregnant mice with hyperthyroidism, alleviating renal damage in offspring, possibly through the Nrf2/heme oxygenase-1/NOD-like receptor thermal protein domain associated protein 3 signaling pathway[73]; Rehmannia polysaccharides can effectively inhibit apoptosis and oxidative damage in rat hippocampal neurons induced by hypoxia-reoxygenation, possibly related to inhibiting the circular RNA 0010729/miR-326 pathway[74]; Rehmannia polysaccharides can reduce vascular calcification induced by vitamin D3 and nicotine in rats, possibly by inhibiting vascular cell apoptosis, thus preventing osteoblastic transformation and calcification of vascular cells[75]; Rehmannia polysaccharides can improve learning and memory impairment induced by scopolamine in mice, with effects related to regulating cholinergic nervous system function, antioxidant stress, and inhibiting inflammatory responses[76]; exercise training combined with Rehmannia polysaccharides can repair neurological function in rats after cerebral ischemia-reperfusion, improving learning and memory abilities, possibly through reducing oxidative stress responses, inhibiting NF-κB pathway activation, and slowing the progression of inflammation[77].2.5 Alkaloids, Phenolic Acids, and IsoflavonoidsStudies have shown that the alkaloids Rehmannine A, Baimantuoluoamide B, and Capparisine C in Rehmannia glutinosa have protective effects against lipopolysaccharide-induced damage in rat renal tubular epithelial NRK-52e cells[26]. Vanillic acid can alleviate palmitic acid-induced fatty degeneration in human liver cancer HepG2 cells by inducing autophagy, indicating its significance in finding potential drugs and targets for the prevention and treatment of non-alcoholic fatty liver disease. Studies have shown that caffeic acid can protect against doxorubicin-induced myocardial injury by reducing oxidative stress, lowering mitochondrial membrane potential, and increasing reactive oxygen species levels in cardiomyocytes H9c2, and by regulating NF-κB signaling pathway[79]. Quercetin-3-O-glucuronide may be the molecular entity exerting hypoglycemic effects, acting directly on the Akt PH region to activate Akt, exerting hypoglycemic effects through the Akt-glycogen synthase kinase-3β pathway[80]. In evaluating the responses of estrogen target cells to a series of isoflavonoid glycosides and aglycones from Genista halacsyi Heldr., it was found that compared to aglycones, daidzein-7-O-β-D-pyranoglucoside and daidzein-7,4′-di-O-β-D-pyranoglucoside have higher estrogen-like and anti-inflammatory activities, suggesting that formulations rich in daidzein-7-O-β-D-pyranoglucoside can be used as alternatives to low-dose estrogen drugs for treating menopausal symptoms[81].2.6 Other Active ComponentsStudies have shown that Qinpi Ethyl Ester has therapeutic effects on lung cancer, gastric cancer, liver cancer, pancreatic cancer, intestinal cancer, prostate cancer, breast cancer, melanoma, brain glioma, multiple myeloma, leukemia, etc., inhibiting cell proliferation, inducing apoptosis, blocking the cell cycle, inhibiting tumor metastasis, suppressing angiogenesis, enhancing immunity, and sensitizing and reducing toxicity[82]. Recent studies have shown that Qinpi Ethyl Ester may inhibit the proliferation, migration, and invasion of human cervical cancer SiHa cells by downregulating FLVCR1-AS1 gene levels[83]; Qinpi Ethyl Ester can inhibit the growth of mouse lung cancer Lewis cell tumors by inducing apoptosis and inhibiting Wnt/β-catenin pathway activation[84]; Qinpi Ethyl Ester has been shown to inhibit the proliferation and stemness of human ovarian cancer SKOV3 cells, while also inducing apoptosis in SKOV3 cells[85]; Qinpi Ethyl Ester can inhibit high glucose-induced renal tubular epithelial cell inflammatory damage by downregulating miR-486-5p expression[86]; Qinpi Ethyl Ester protects human retinal pigment epithelial cell line ARPE-19 from oxidative damage by upregulating intracellular antioxidant enzyme activity or the expression of antioxidant proteins[87].Studies have shown that 3,4-dihydroxyacetophenone has anti-inflammatory and antioxidant effects, and is expected to become an effective drug for treating acute lung injury[88]; 3,4-dihydroxyacetophenone may lower triglyceride levels in liver cells and liver tissues through the AMPK pathway[89]; 3,4-dihydroxyacetophenone effectively inhibits the inflammatory response of lipopolysaccharide-induced macrophages RAW264.7 by inhibiting NF-κB nuclear translocation[90]; 3,4-dihydroxyacetophenone has protective effects on myocardium[91]; 3,4-dihydroxyacetophenone may relax pulmonary arterial smooth muscle cells by opening potassium channels, which may be one of its important mechanisms for lowering pulmonary arterial hypertension[92]; 3,4-dihydroxyacetophenone’s mechanism of inhibiting platelet aggregation may be related to inhibiting thromboxane B2 release in the arachidonic acid metabolic pathway[93].5 Pharmacological Basis Research under Formula CombinationsLiu Shaobo et al.[100] used ultra-performance liquid chromatography-quadrupole-time of flight-mass spectrometry (UPLC-Q-TOF-MS) technology to replicate a kidney yin deficiency model in rats using hydrocortisone, finding that Zhibai Dihuang Wan can effectively alleviate kidney yin deficiency, and the active components belonging to Rehmannia glutinosa in the serum of the treated rats mainly include Hairy Flower Sugar, Rhamnose, Maltose, and Berberine sulfate glucuronide. Liu Kaixin et al.[101] used UPLC-Q-TOF-MS technology to replicate a bone marrow suppression model in rats using recombinant human granulocyte-stimulating factor, finding that Shengyu Decoction significantly increased the body weight, hemoglobin, red blood cell count, and platelet count in bone marrow suppression rats; under effective conditions, a total of 26 prototype components and 20 metabolites were characterized, among which the blood components belonging to Rehmannia glutinosa included Rehmannin, Rehmannin metabolites, Hairy Flower Sugar glycoside metabolites, and Rehmannin D metabolites. Duan et al.[102] used UPLC-Q-TOF-MS technology to study the effects of Taohong Siwu Decoction on SD rats, identifying a total of 39 active components and 90 metabolites in the blood and brain tissues of the rats, among which the prototype components derived from Rehmannia glutinosa included焦地黄苯乙醇苷B1, Astragalin, and Rehmannin D. Wang et al.[103] used UPLC-Q-TOF-MS technology to study the effects of Zuogui Wan on SD rats, detecting prototype components from Rehmannia glutinosa in rat serum samples, including β-D-ribofuranuronic acid methyl ester triacetate, 5-hydroxymethyl-2-furfural glucuronide, and dihydro-5-hydroxymethyl-2-furfural glucuronide, with metabolites being 3-hydroxy-2,6,6-trimethyl-1-cyclohexene-1-carboxylic acid. Yang Huajie et al.[104] used UPLC-Q-TOF-MS technology to find that in SD rats treated with Liuwei Dihuang Wan, a total of 32 migrating components were detected in rat serum, among which the chemical components derived from Rehmannia glutinosa included 8-epiloganic acid, Gardenia glycoside, acetyl Rehmannin, Hairy Flower Sugar glycoside, Rehmannin, Iso-Rehmannin, oleanolic acid, mannitol, dibutyl phthalate, and protocatechuic acid. Compounds absorbed into the human/animal body in the form of prototype components or metabolites are likely to be the pharmacological basis for regulating diseases, so under effective conditions, compounds detected in animals through high-resolution separation and identification techniques are more worthy of attention and further pharmacological activity research.4 Conclusion and OutlookThis study reviews the research progress on the chemical composition, pharmacological effects of active components, and the pharmacological basis under the conditions of formula combinations of Rehmannia glutinosa, finding that Rehmannia glutinosa mainly contains various types of compounds such as iridoids, phenolic compounds, and phenylethanoid compounds. The pharmacologically active components in Rehmannia glutinosa, including Rehmannin D, Rehmannin, Peach Leaf Coral Glycoside, Rehmannioside, Ginsenoside, Rehmannia bitter glycoside, Echinacoside, Hairy Flower Sugar Glycoside, Rehmannia polysaccharides, Qinpi Ethyl Ester, 3,4-dihydroxyacetophenone, and 5-hydroxymethylfurfural, may be the chemical basis for its therapeutic effects. The pharmacological effects are mainly reflected in blood sugar lowering, anti-tumor, improving liver fat, antidepressant, improving cognitive impairment, and having estrogen-like activity. The uncertainty of effective components and unclear mechanisms of action have always been a bottleneck restricting the modernization of traditional Chinese medicine. In recent years, the continuous development of serum pharmacochemistry and high-resolution separation and identification technologies has accelerated research related to the pharmacological basis of traditional Chinese medicine. Serum pharmacochemistry has achieved a leap from in vitro to in vivo research on active components of traditional Chinese medicine, allowing for the characterization of compounds that exert effects through absorption into the serum and further pharmacokinetic and mechanism analysis, deepening researchers’ understanding of effective components of traditional Chinese medicine. Further practice and exploration in related research are needed. Traditional Chinese medicine has characteristics of “holism, multi-targeting, and synergy,” and research on the pharmacological basis of traditional Chinese medicine must align with the fundamental theories of traditional Chinese medicine, rather than focusing solely on single active components. The multi-level data integration concept of systems biology is closely related to the “holistic view” of traditional Chinese medicine, encompassing genomics, transcriptomics, proteomics, metabolomics, and network pharmacology, achieving new breakthroughs in the evaluation of efficacy and mechanisms of traditional Chinese medicine formulas. Therefore, further developing systems biology research methods may help advance research on the pharmacological basis of traditional Chinese medicine, promoting traditional Chinese medicine to the world stage and better serving the health of all humanity.Conflict of InterestAll authors declare no conflict of interest.References (omitted) Source: Ge Nan, Yan Guangli, Sun Hui, Wang Xijun. Research Progress on the Pharmacological Basis of Rehmannia Glutinosa [J]. Chinese Herbal Medicine, 2023, 54(1):292-302.