Click “Read the original text” to view the journal content
【Authors】Zhao Zhongting, Zhao Yikun, Zhu Tiantian, Xing Jiaming, Bu Xiaomei, Zhang Yanfeng, Yan Xingke
【Abstract】Objective: To observe the effects of acupuncture with fine needles on the neuro-electrophysiological activity characteristics in the hippocampal CA1 and CA3 regions of rats with post-traumatic stress disorder (PTSD) model. Methods: Fifty Sprague-Dawley (SD) rats were randomly divided into a blank group, model group, restraint group, Western medicine group, and acupuncture group, with 10 rats in each group. Except for the blank group, the other four groups were subjected to a composite stress method for modeling. Simultaneously, the Western medicine group was given paroxetine hydrochloride by gavage, the acupuncture group received acupuncture intervention, and the restraint group was subjected to restraint without any treatment. After 14 days of intervention, in vivo multi-channel recordings of neuronal cluster discharges in the hippocampal CA1 and CA3 regions were performed to analyze the interspike interval (ISI) and power spectral density (PSD) and to create graphs. Results: Compared with the blank group, the ISI in the CA1 and CA3 regions of the model group and restraint group was prolonged, and the PSD concentrated distribution area shifted downward (P<0.05 or P<0.01); compared with the restraint group, the ISI in the CA1 and CA3 regions of the Western medicine group and acupuncture group was shortened, and the PSD concentrated distribution area shifted upward (P<0.05 or P<0.01); there was no statistical difference in ISI and PSD concentrated distribution area between the acupuncture group and the Western medicine group (both P>0.05). Conclusion: Both acupuncture with fine needles and paroxetine hydrochloride intervention can significantly regulate the discharge patterns of neuro-electrophysiological activity characteristics in the hippocampal CA1 and CA3 regions of rats with PTSD model, which may be one of the mechanisms by which acupuncture promotes recovery from PTSD.
【Reference Format】Zhao ZT, Zhao YK, Zhu TT, et al. Effects of acupuncture on neuro-electrophysiological activities in hippocampal CA1 and CA3 areas of rats with post-traumatic stress disorder. J Acupunct Tuina Sci, 2019, 17(2): 67-73
Post-traumatic stress disorder (PTSD) refers to a stress response that gradually worsens over time due to the body experiencing threatening and catastrophic psychological trauma, classified as a mental psychological syndrome [1]. Natural disasters and social events such as traffic and production accidents have increased the incidence of PTSD. Currently, commonly used medications and psychological methods for treating PTSD have significant adverse reactions and limitations [2-3]. Our research team found that traditional Chinese medicine acupuncture treatment for PTSD has significant advantages and has rapidly gained widespread attention due to its simplicity, ease of use, and lack of adverse reactions and side effects.
The hippocampus is a high-level regulatory center for stress responses and can directly participate in the formation of PTSD [4]. Damage to the hippocampus can lead to the loss of its regulatory feedback on stress, and decreased neuronal activity can further exacerbate stress responses [5]. Among the various structures of the hippocampus, the CA1 and CA3 regions are most closely related to PTSD [6]. Therefore, our research team used in vivo neural signal synchronous recording technology to obtain information on the interspike interval (ISI) and power spectral density (PSD) of neuronal cluster discharges in the CA1 and CA3 regions of rats with PTSD model to preliminarily explore the central nervous mechanism of acupuncture intervention in PTSD.
1 Experimental Animals and Materials
1.1 Experimental Animals
Fifty SPF-grade healthy male Sprague-Dawley (SD) rats aged 2 months [provided by the Research Experiment Center of Gansu University of Chinese Medicine, Certificate No.: 62001000000091, License No.: SCXK(Gan)2011-0001], with a body weight of (200±20) g. The rats were acclimatized for 7 days, during which they had free access to food and water. The environmental temperature was (23±2)℃, and the relative humidity was (60±10)%; a 12 h/12 h light cycle was maintained (8:00-20:00 light); the rats were handled for 3 minutes daily to avoid startling them. Animal handling strictly followed the “Guidelines for the Care and Use of Laboratory Animals”.
1.2 Experimental Instruments and Equipment
Electric shock and confinement modeling box and power supply, circuit (self-made in our laboratory); brain stereotaxic apparatus (Shenzhen Ruivode Life Science Technology Co., China); Cerebus multi-channel data acquisition system (including microelectrode array, preamplifier, processor, independent regulated power supply, PC workstation, etc., Blackrock Microsystem Inc., USA); MEA 16-channel (4×4) acute microelectrode array (MicroProbes, USA); disposable sterile acupuncture needles (diameter 0.25 mm, length 25 mm, Suzhou Medical Supplies Factory Co., China).
1.3 Experimental Drugs and Reagents
10% chloral hydrate (Batch No.: 20121106, Shanghai Zhanyun Chemical Co., China) and 25% urethane (Batch No.: 20130609, China National Pharmaceutical Group Chemical Reagents Co., China) mixed anesthetic in a 1:1 ratio; paroxetine hydrochloride (Batch No.: 150104, Zhejiang Jianfeng Pharmaceutical Co., China) solution (2 mg/mL).
2 Experimental Methods
2.1 Grouping and Intervention
Using a random number table method, the 50 rats were divided into a blank group, model group, restraint group, Western medicine group, and acupuncture group, with 10 rats in each group, and each animal was housed in a single cage. Since a maximum of 6 rats could be modeled simultaneously, after grouping, all rats were randomly divided into multiple batches of 6 for sequential experiments until in vivo multi-channel signal recording was completed. The intervention methods for each group were as follows:
Blank group: No modeling, no restraint, no treatment.
Model group: PTSD modeling, no restraint, no treatment.
Restraint group: PTSD modeling, restrained but not treated, with the same duration of restraint as the acupuncture and Western medicine groups.
Western medicine group: PTSD modeling, restrained, and then given paroxetine hydrochloride solution by gavage at a dose of 5 mL/(kg•bw). The drug solution was administered uniformly within 1 minute, and after withdrawing the gavage needle, restraint was continued for 4 minutes.
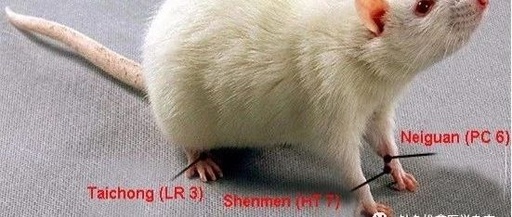
Acupuncture group: PTSD modeling, restrained, and acupuncture was performed at Baihui (Du20), Neiguan (PC6), Shenmen (HT7), and Taichong (LR3) according to “Experimental Acupuncture” [7]. After routine disinfection, the Baihui point was punctured backward 4-5 mm using the lifting and thrusting technique, followed by oblique punctures at Neiguan, Shenmen, and Taichong for 2-3 mm, leaving the needles in place. During needle retention, basic needling techniques were applied for 1 minute per needle, and after 4 minutes, all needles were removed, and dry cotton swabs were used to apply pressure to stop bleeding before returning the rats to their cages. When acupuncture was performed on the limb acupoints, the left and right sides were alternated every other day.
All restraint or treatment was performed once daily for a total of 14 days.
2.2 PTSD Model Replication
Referring to literature [8], the PTSD model was replicated in all groups except the blank group using a composite stress method, with 6 rats modeled simultaneously in each batch. The modeling box was cleaned, and an alternating current voltage of 60 V and a current of 48 mA (8 mA per box) were set. Each rat was placed individually in the box, deprived of light and confined for 30 minutes; during this time, the power supply was randomly turned on to apply 4 seconds of electrical stimulation to the rat’s footpad, repeated 30 times with random intervals. This electric shock and confinement method was applied once daily for 6 consecutive days. On the 7th day, a 70 g weight was attached to the rat’s neck, and the rat was placed in a water tank at a temperature of (28±2)℃ for forced swimming. When the rat became exhausted, stopped struggling, and began to sink, it was immediately removed and placed in a warm, dry area to dry off before returning to its cage. The blank group received no treatment and was raised conventionally.
2.3 Stereotaxic Positioning and Craniotomy
Using a dose of 5 mL/(kg•bw), the mixed anesthetic was administered via intraperitoneal injection to anesthetize the rats. When the rats fell over, exhibited muscle relaxation, stable breathing, and no response to footpad stimulation, anesthesia was considered successful. After successful anesthesia, the rats were placed in the brain stereotaxic apparatus, and their heads were fixed with a nose clamp to ensure normal ventilation and adjust height so that the anterior fontanelle and sagittal suture were at the same level; the bilateral ear bar scales were adjusted to center the rat’s head and fixed, and the local area was shaved and disinfected. A surgical knife was used to make a linear incision in the skin of the skull along the midline from the midpoint between the eyes to the midpoint between the front edges of the ears. Blunt dissection was performed with straight forceps to separate the subcutaneous tissue, peel off the periosteum, and hydrogen peroxide was used to remove residual material from the skull surface, followed by saline immersion to fully expose the skull and the anterior fontanelle, midline, and sagittal suture as positioning landmarks.
Referring to the “Stereotaxic Atlas of the Rat Brain” [9] and literature [10], the CA1 and CA3 regions of the hippocampus were located on the left and right sides of the midline, respectively. Under a microscope, a projection area of approximately 3 mm × 3 mm was defined at the skull surface corresponding to the CA1 and CA3 regions, and multiple holes were drilled at the edges of the area to a depth sufficient to penetrate the skull without damaging the dura mater. After drilling, the skull was removed with ophthalmic forceps, and the dura mater was peeled off. After exposing the cerebral cortex, saline was used to moisten the area in preparation for electrode insertion.
2.4 Electrode Implantation and Signal Collection
Using a microelectrode manipulator installed on the stereotaxic apparatus, the MEA was fixed, and the dura mater was set as the longitudinal axis “0” position. Based on the digital display module readings, the microelectrode manipulator was used to slowly push the MEA into the CA1 and CA3 regions of the hippocampus, and the electrode ground wire was buried in the surrounding subcutaneous tissue.
After opening the multi-channel neural signal recording system, the Central software was set to a sampling frequency of 20 kHz, a filtering range of 0.3-7.5 kHz, and an effective threshold potential of -63 μV. After ensuring that the signals were not disturbed, real-time data was recorded for 180 seconds when the electrical activity was rich and stable. The collected electrical signals were converted into ISI using Neuro Explorer software to obtain the average ISI over 180 seconds and generate scatter plots; spectral signals were recorded synchronously, and the fast Fourier transform (FFT) of the signal autocorrelation function was calculated to derive the PSD and export the spectral graph. After signal recording, the animals were euthanized and disposed of humanely.
2.5 Statistical Processing
Statistical processing was performed using SPSS Statistics 19.0 software, setting equal intervals for each group of data. Measurement data results were expressed as mean ± standard deviation (±s), and inter-group comparisons were conducted using one-way ANOVA. The least significant difference (LSD) method was used when variances were equal, and Tamhane’s T2 test was used when variances were unequal. A P-value ≤ 0.05 was considered statistically significant.
3 Experimental Results
3.1 General Condition and Behavioral Testing
After modeling, compared with the blank group, the model group rats showed significantly reduced activity and food intake, were easily startled, huddled together, and had messy fur; behavioral testing revealed a decrease in the number of crossings in the open field test and a lower refusal reflex score, confirming successful modeling. After treatment, compared with the restraint group, the Western medicine group and acupuncture group rats had neater fur, were generally active, agile, in good spirits, and were more docile, not easily startled, with normal food intake, although some still exhibited heightened alertness, and some rats in the Western medicine group showed drowsiness.
3.2 ISI
3.2.1 Distribution
After modeling, compared with the blank group, the discharge interval sequence in the model group was significantly prolonged, and the number of discharge pulses decreased, but there was no significant change in the restraint group compared to the model group. After treatment, both the Western medicine group and acupuncture group showed a significant shortening of the discharge interval sequence and an increase in the number of discharge pulses compared to the restraint group, but there was no significant difference between the two treatment groups, indicating that both therapies could regulate the ISI distribution, but with no significant difference (Table 1).
3.2.2 Data Results
After modeling, compared with the blank group, the ISI in the model group was prolonged (CA1 region P<0.05, CA3 region P<0.01), indicating that composite stress could prolong the discharge interval in this brain region, leading to abnormal ISI, with a greater impact on the CA3 region; there was no statistical difference between the restraint group and the model group (both regions P>0.05), indicating that restraint did not affect ISI. Compared with the restraint group, after treatment, the ISI in the Western medicine group and acupuncture group was shortened (both groups CA1 region P<0.05, both groups CA3 region P<0.01), indicating that both therapies could regulate ISI, with a greater impact on the CA3 region; there was no statistical significance between the Western medicine group and acupuncture group (both regions P>0.05), indicating that both therapies had a comparable effect on regulating ISI. See Figure 2 for details.
3.3 PSD
3.3.1 Distribution
After modeling, compared with the blank group, the PSD concentrated distribution area in the model group shifted downward, but there was no significant change in the restraint group compared to the model group. Compared with the restraint group, the PSD concentrated distribution area in the Western medicine group and acupuncture group shifted upward after treatment, indicating that both therapies could positively regulate the PSD, but there was no significant difference between the Western medicine group and acupuncture group, indicating that both therapies could regulate the PSD distribution range. See Table 2 for details.
3.3.2 Data Results
After modeling, compared with the blank group, the PSD in the model group decreased (CA1 region P<0.05, CA3 region P<0.01), indicating that composite stress could cause a decrease in PSD and abnormalities, with a greater impact on the CA3 region. There was no statistical significance between the restraint group and the model group (both regions P>0.05), indicating that restraint did not affect PSD. Compared with the restraint group, both the Western medicine group and acupuncture group showed an increase in PSD after treatment (both groups CA1 region and Western medicine group CA3 region P<0.05, acupuncture group CA3 region P<0.01), indicating that both therapies could promote the recovery of PSD, with acupuncture having a more significant effect on the CA3 region; there was no statistical difference between the Western medicine group and acupuncture group (both regions P>0.05), indicating that both therapies had no significant difference in regulating PSD. See Figure 3 for details.
4 Discussion
The hippocampus is one of the brain regions most significantly affected by stress regulation. During the stress response, the sympathetic adrenal medullary system can cause prolonged elevation of norepinephrine levels [11], promoting the excessive release of excitatory amino acids in the hippocampus, leading to intracellular calcium overload, which in turn causes calcium overload and increased release in organelles such as mitochondria and endoplasmic reticulum, and interacts with calmodulin, resulting in decreased neuronal activity and dysfunction in the hippocampus [12]; increased corticotropin-releasing hormone (CRH) is a key link in the activation of the hypothalamic-pituitary-adrenal (HPA) axis, with the hypothalamus increasing CRH secretion, leading to increased secretion of adrenocorticotropic hormone and glucocorticoids, resulting in abnormal activation of the HPA axis and causing PTSD-related symptoms. Studies have found that CRH expression is higher in the CA1 and CA3 regions of the hippocampus in PTSD rats, indicating that among the various structures of the hippocampus, the CA1 and CA3 regions are most closely related to PTSD [6]. Sousa N et al. [13] found that unpredictable chronic stress not only shortened the dendritic length of pyramidal cells in the CA3 region of the hippocampus by 28% but also shortened the dendritic length of pyramidal cells in the CA1 region by 13%, although not as much as in the CA3 region, but sufficient to confirm significant changes in the CA1 and CA3 key brain regions during the stress response. The hippocampal region is an important part of the brain for information processing [14], and neuro-electrophysiology suggests that neurons are connected by synapses, and information exchange is achieved through the discharge time and spatial sequence of many action potentials, forming the information coding characteristics of the neural center based on the complex network structure of numerous neurons in the cerebral cortex. Research based on the characteristics of neurobiological electrical signals is an innovative field in brain function research [15-16].
Neuronal cluster discharge in vivo synchronous multi-channel recording technology is a key technology for studying brain neural networks [17]. By applying this technology, the traditional recording of single neuron activity has evolved into synchronous recording of neuronal cluster discharge activities, allowing for precise analysis of the relationships between the time, frequency, and space of neural electrical signals, recording high-resolution spatiotemporal information carried during local area transmission, providing new ideas for studying the temporal and spatial connections of different brain regions and nuclei. Currently, in vivo multi-channel technology has been applied in studies of higher animals related to learning, memory, sensation, movement, and other life phenomena and disease changes closely related to the nervous system [18], becoming an important tool for revealing the synergistic effects of neural electrical signals at different levels [19]. The information coding characteristics of neuronal clusters are reflected not only in discharge frequency but also in the main features of action potentials concentrated in peak potentials, making peak potential synonymous with action potentials, and ISI has become a research object in numerous neuro-electrophysiological studies [20]; some scholars believe that the ISI of electrophysiological activity in organisms is the sequential coding of information transmission, and sequence analysis is recognized as a major element carrying biological information, playing an important role in neural information coding [21-22]. Processing of cluster discharge signals in the CA1 region of the hippocampus in mice revealed that the discharge waves of pyramidal cells in the CA1 region exhibited short and sharp peaks and decreasing peaks and interval peaks, indicating periodic characteristics of hippocampal discharge [23]. In estimating the power spectrum of field potentials, the PSD of random signals can describe the power characteristics of signals as they change with discharge frequency [14], belonging to frequency domain coherence analysis, which is also an extremely important piece of information for processing electrical signals [21].
The results of this study indicate that acupuncture with fine needles can shorten the abnormal action potential ISI in the CA1 and CA3 regions of PTSD model rats and increase the number of discharge pulses; it promotes the upward shift of the PSD concentrated distribution area, restoring abnormal PSD. The effect on the CA3 region is significant, and there is no significant difference compared to paroxetine hydrochloride. This indicates that acupuncture with fine needles can effectively regulate the basic form of abnormal neuro-electrophysiological neural network coding, which may be a relevant mechanism by which acupuncture promotes recovery from PTSD, providing experimental evidence for the effectiveness of traditional Chinese medicine acupuncture treatment for PTSD.
To cite this article: Zhao ZT, Zhao YK, Zhu TT, et al. Effects of acupuncture on neuro-electrophysiological activities in hippocampal CA1 and CA3 areas of rats with post-traumatic stress disorder. J Acupunct Tuina Sci, 2019, 17(2): 67-73
This article is sourced from “Acupuncture and Tuina Science (English Edition)” 2019, Issue 2
Click “Read the original text” to view the journal content