Chief Commentator of The Paper, Shen Bin
The incident of “a confinement center fined 30,000 for adding Codonopsis (Dangshen) to chicken soup” has gone viral. The market supervision administration of Haishu District, Ningbo City, conducted an on-site inspection of a certain confinement center and found that the center added Codonopsis to the chicken soup for postpartum women. Upon investigation, Codonopsis is not classified as a substance that is both a food and a medicinal herb, thus the center was fined 30,000.
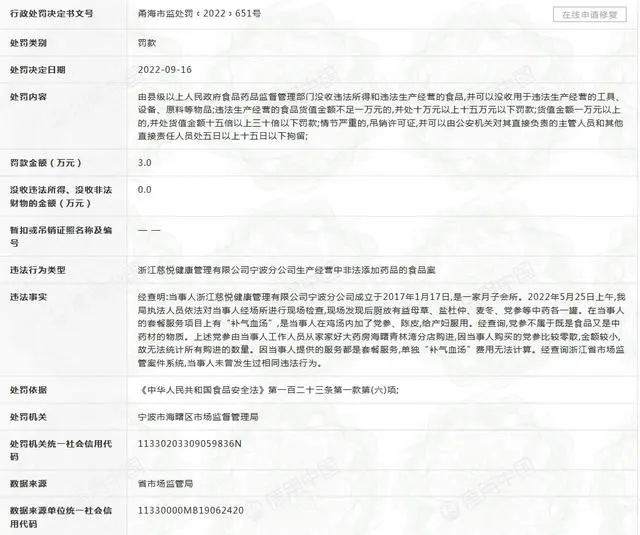
Adding Codonopsis to chicken soup results in a fine of 30,000; can we still enjoy making soup? Many netizens believe that many medicinal herbs are “homologous in food and medicine”. If adding Codonopsis incurs a fine, will adding jujube (Da Zao), ginger (Sheng Jiang), or adzuki beans (Chi Xiao Dou), which are also considered “medicinal”, also lead to penalties? Some even referenced a related notice published in 2021, arguing that Codonopsis has already been included in the “homologous food and medicine” list, and thus there is no violation.
In fact, this incident reflects the current chaos in the “homologous food and medicine” market. The marketing of traditional medicine and health preservation is rampant, but which practices are compliant and which are misleading? Consumers should not be at risk to their health after spending large amounts of money.
“Homologous food and medicine” is an important concept in traditional Chinese medicine (TCM), but it is “not a catch-all”; it cannot be indiscriminately applied. It falls under a special category of food and drug regulation. The Food Safety Law clearly states that “medications must not be added to food products”, which includes the indiscriminate addition of medicinal herbs. However, considering the dietary traditions of the Chinese people, to promote and protect TCM, it is also specified that food can “include substances that are traditionally both food and medicinal herbs”.
Substances that are both food and medicinal herbs are clearly defined. In 2002, the former Ministry of Health issued a notice on further regulating the management of health food ingredients, identifying 87 substances that are both food and medicinal herbs, and in 2019, six more were added. Common ingredients in Chinese cuisine that fall under “homologous food and medicine” are mostly covered, such as honey (Feng Mi), clove (Ding Xiang), yam (Shan Yao), hawthorn (Shan Zha), licorice (Gan Cao), chrysanthemum (Ju Hua), and almond (Xing Ren). Ingredients on this list can be added to daily foods; those not on the list cannot be added.
Some netizens questioned: will using “medicinal” ginger (Jiang) and garlic (Suan) in cooking lead to penalties in the future? In fact, ginger has been included in the “homologous food and medicine” directory since 2002; while garlic, although not on the list, is one of the 68 legally recognized spices and complies with national standards (GB/T12729.1-2008 “Names of Spices and Condiments”). Its legal use is completely unproblematic.
As for the classification of Codonopsis, the situation is more complex. In January 2020, the National Health Commission and the State Administration for Market Regulation jointly issued a notice on conducting pilot management of Codonopsis and eight other substances as traditional substances that are both food and medicinal herbs. However, the “pilot work” is not a nationwide initiative; it is based on specific pilot plans proposed by provincial health commissions in conjunction with market regulation bureaus (departments, commissions) according to local conditions, which are then submitted for approval. The incident occurred in Zhejiang Province, which did not apply for a pilot program for Codonopsis, so the addition of Codonopsis to commercially produced chicken soup is considered illegal in that locality (of course, adding it in home cooking does not fall under “production and operation activities” and is not subject to this restriction).
Why can’t medicinal herbs be added arbitrarily to ingredients processed by enterprises? Aren’t medicinal herbs “great tonics”?
This is actually a misunderstanding. TCM emphasizes syndrome differentiation and treatment, and formulas require a consideration of the monarch, minister, assistant, and envoy. Even “tonic herbs” cannot be taken indiscriminately. There is a saying that “all medicines have some toxicity”; of course, the “toxicity” referred to in TCM is more about the inherent properties of the herbs, meaning that a specific medicinal herb may not be suitable for everyone. If medicinal herbs are added to food without specific diagnosis and provided to an unspecified group, it may lead to adverse consequences and even public health risks. In 2003, during the SARS outbreak, some businesses produced “Ban Lan Gen beer” and “Ban Lan Gen dishes”, which were immediately banned.
Codonopsis is generally considered a medicinal herb with a neutral flavor, but due to its “sweet tonifying” properties, it is not suitable for those with excess heat conditions, as it is clearly stated in the pharmacopoeia that “those with excess heat should avoid it”. For example, postpartum women with symptoms of constipation and other signs of excess heat are not suitable for consuming Codonopsis. Moreover, Codonopsis has compatibility contraindications; the famous TCM texts “Eighteen Contradictions and Nineteen Avoidances” include the prohibition of “various ginsengs against Bai Lian”. Not differentiating the constitution of postpartum women and adding Codonopsis to chicken soup under the guise of “tonifying qi and blood” marketing is not only inconsistent with TCM knowledge but also violates modern food and drug regulatory systems.

Chinese traditional medicine is broad and profound, but it is precisely because of this that some businesses take advantage of the “TCM” banner to muddle through, lacking pharmacological understanding and differentiation of constitution, blindly boasting about “great tonics”, “yang-boosting”, and “yin-nourishing”, even openly promoting so-called “therapeutic effects”, indiscriminately adding medicinal herbs to ordinary ingredients to elevate their status, leaving consumers confused.
In response to the chaos in the “homologous food and medicine” industry, in November 2021, the National Health Commission issued the “Regulations on the Management of the Directory of Substances that are Both Food and Medicinal Herbs According to Tradition” (hereinafter referred to as “Regulations”), which provides clear specifications. The “Regulations” formally define “homologous food and medicine” as “food and drug substances”, where “food and drug substances” refer to those traditionally used as food and listed in the “Pharmacopoeia of the People’s Republic of China”.
The “Regulations” also clarify the entry threshold for “food and drug substances”: 1. There is a traditional habit of consumption as food; 2. It has been included in the “Pharmacopoeia of China”; 3. Safety assessments have not revealed food safety issues; 4. It complies with relevant laws and regulations on the protection of medicinal herb resources, wildlife protection, and ecological protection. According to this entry standard, only those medicinal herbs that have a traditional habit of being consumed as food may enter the directory, and for newly included substances, they must be listed in the food safety risk monitoring plan by the provincial health commission. This entry standard is both open and cautious, responsible for the health of the people while leaving room for the development of the “homologous food and medicine” industry.
It should be noted that health foods, dietary supplements, and medicinal diets often exist in a regulatory gray area. On one hand, the market prospects are huge and profits are substantial; on the other hand, due to the lack of clear rules, they can become a disaster zone for consumer interests, potentially causing financial loss and health risks.
In this particular case, since the local Zhejiang authorities did not conduct a pilot program for Codonopsis as a “food and drug substance”, the confinement center, as a producer and operator, adding Codonopsis to chicken soup naturally crossed the red line of the Food Safety Law. The larger issue behind this is: how to streamline the regulatory mechanisms for health foods, new resource foods, and “food and drug substances”? How to achieve the integration of traditional medicine and modern food regulation?
Senior Editor of this issue, Xing Tan
Recommended Reading





