—————“Only the fittest survive”
Hello everyone! Welcome to the Little Pharmacy Channel
With a few months left until the postgraduate entrance exam, I will update the high-frequency points of the four major pharmaceutical subjects daily. Whether eating, watching dramas, chatting, or before bed, make good use of your fragmented time and follow along with Little Pharmacy for a song’s duration to accompany you on your study journey!Let’s check in together!
【High-Frequency Point 18】Definition, Characteristics, Preparation, Quality Inspection, and Examples of Powder Formulations
1. Definition Powders (散剂, sàn jì) refer to dry powder formulations made by grinding and uniformly mixing drugs with suitable excipients, which can be classified into oral powders and topical powders.2. Characteristics (1) Small particle size, large specific surface area, easy dispersion, and rapid onset of action. (2) When applied externally, they cover a large area and also have protective and astringent effects. (3) Simple preparation process, easy dosage control, and convenient for special populations such as infants and the elderly. (4) Convenient for packaging, storage, transportation, and carrying. (5) Adverse effects such as hygroscopicity, chemical reactivity, odor, irritability, and volatility due to the high dispersion of powders.3. Preparation of PowdersThe general process for preparing powders is shown in the image below: [Factors affecting mixing effectiveness and measures to prevent uneven mixing]: ① Proportion of components: Two drug powders with similar states and particle sizes can be mixed uniformly after a certain time, but if there is a significant difference in quantity or proportion, it will be difficult to mix evenly. In this case, the equal quantity incremental mixing method (also known as the preparation method) should be used, where a small amount of the drug is finely ground and mixed with an equal volume of other powdered drugs, gradually increasing the quantity until fully mixed, followed by sieving.② Density properties: Two drug powders with similar densities are easier to mix, but if there is a large density difference, the lighter powder should be placed in the mixing container first, followed by the heavier powder to avoid the lighter powder floating on top or being blown away, while the heavier powder settles at the bottom and is difficult to mix.③ Adsorption and charge properties: Some drug powders have adsorption properties to mixing equipment, affecting mixing and causing losses. Generally, the larger and less adsorptive powders or excipients should be placed at the bottom, while the smaller and more adsorptive ones should be added later. Powders that become charged due to mixing friction often hinder uniform mixing; a small amount of surfactant can be added to overcome this, and lubricants can also be used as antistatic agents, such as adding 0.25% to 0.5% magnesium stearate to aspirin powder for antistatic effects.④ If the powder contains liquid or hygroscopic components, appropriate measures should be taken before mixing to ensure uniformity.⑤ Components that can form low eutectic mixtures: When two or more drugs are mixed in certain proportions, the wetting and liquefaction phenomenon that occurs at room temperature is called the low eutectic phenomenon. This phenomenon is detrimental to the mixing of components. For example, mixing 45% camphor (melting point 179°C) with 55% salol (melting point 42°C) results in a low eutectic mixture with a melting point reduced to 6°C, which liquefies at room temperature.4. Quality Inspection of Powders (Weight Variation)See Table 4 for details (This is also something to remember)
[Factors affecting mixing effectiveness and measures to prevent uneven mixing]: ① Proportion of components: Two drug powders with similar states and particle sizes can be mixed uniformly after a certain time, but if there is a significant difference in quantity or proportion, it will be difficult to mix evenly. In this case, the equal quantity incremental mixing method (also known as the preparation method) should be used, where a small amount of the drug is finely ground and mixed with an equal volume of other powdered drugs, gradually increasing the quantity until fully mixed, followed by sieving.② Density properties: Two drug powders with similar densities are easier to mix, but if there is a large density difference, the lighter powder should be placed in the mixing container first, followed by the heavier powder to avoid the lighter powder floating on top or being blown away, while the heavier powder settles at the bottom and is difficult to mix.③ Adsorption and charge properties: Some drug powders have adsorption properties to mixing equipment, affecting mixing and causing losses. Generally, the larger and less adsorptive powders or excipients should be placed at the bottom, while the smaller and more adsorptive ones should be added later. Powders that become charged due to mixing friction often hinder uniform mixing; a small amount of surfactant can be added to overcome this, and lubricants can also be used as antistatic agents, such as adding 0.25% to 0.5% magnesium stearate to aspirin powder for antistatic effects.④ If the powder contains liquid or hygroscopic components, appropriate measures should be taken before mixing to ensure uniformity.⑤ Components that can form low eutectic mixtures: When two or more drugs are mixed in certain proportions, the wetting and liquefaction phenomenon that occurs at room temperature is called the low eutectic phenomenon. This phenomenon is detrimental to the mixing of components. For example, mixing 45% camphor (melting point 179°C) with 55% salol (melting point 42°C) results in a low eutectic mixture with a melting point reduced to 6°C, which liquefies at room temperature.4. Quality Inspection of Powders (Weight Variation)See Table 4 for details (This is also something to remember)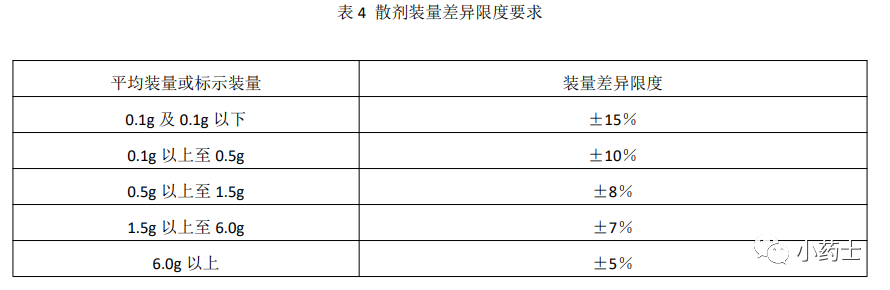 5. Examples of Typical Formulations (Just for understanding)(1) Powder formulation containing low eutectic components (prickly heat powder)[Formula] Talcum powder 67.7% Salicylic acid 1.4% Zinc oxide 6.0% Boric acid 8.5% Sublimed sulfur 4.0% Thymol 0.6% Menthol 0.6% Peppermint oil 0.6% Camphor 0.6% Starch 10%[Preparation] First, grind thymol, menthol, and camphor to form a low eutectic mixture, mix with peppermint oil, and separately mix and grind sublimed sulfur, salicylic acid, boric acid, zinc oxide, starch, and talcum powder in a ball mill to a fine powder, then sieve through a 100-120 mesh screen. Place this fine powder in a mixing cylinder (with a spray device), spray in the low eutectic mixture containing peppermint oil, mix uniformly, and sieve to obtain the final product.[Notes] ① In this product, thymol, menthol, and camphor undergo low eutectic mixing during preparation, utilizing this phenomenon to ensure uniform mixing with other drugs; ② Talcum powder, zinc oxide, etc., should be sterilized before use; ③ This product has hygroscopic, anti-itch, and astringent effects, suitable for heat rash, prickly heat, etc.(2) Dilution powder: Dilution powder is a diluted powder made by adding a certain amount of filler to a small dose of highly toxic drugs for the next step of preparation. The dilution factor is determined by the dosage: a dosage of 0.1-0.01g can be made into a 10-fold powder (i.e., a powder mixed uniformly with 9 parts diluent and 1 part drug), 0.01-0.001g can be made into a 100-fold powder, and below 0.001g should be made into a 1000-fold powder.The highlighted parts are key points; the rest is for your understanding!That’s all for today; we will continue tomorrow!Welcome to follow Little Pharmacy, let’s explore pharmacy together!
5. Examples of Typical Formulations (Just for understanding)(1) Powder formulation containing low eutectic components (prickly heat powder)[Formula] Talcum powder 67.7% Salicylic acid 1.4% Zinc oxide 6.0% Boric acid 8.5% Sublimed sulfur 4.0% Thymol 0.6% Menthol 0.6% Peppermint oil 0.6% Camphor 0.6% Starch 10%[Preparation] First, grind thymol, menthol, and camphor to form a low eutectic mixture, mix with peppermint oil, and separately mix and grind sublimed sulfur, salicylic acid, boric acid, zinc oxide, starch, and talcum powder in a ball mill to a fine powder, then sieve through a 100-120 mesh screen. Place this fine powder in a mixing cylinder (with a spray device), spray in the low eutectic mixture containing peppermint oil, mix uniformly, and sieve to obtain the final product.[Notes] ① In this product, thymol, menthol, and camphor undergo low eutectic mixing during preparation, utilizing this phenomenon to ensure uniform mixing with other drugs; ② Talcum powder, zinc oxide, etc., should be sterilized before use; ③ This product has hygroscopic, anti-itch, and astringent effects, suitable for heat rash, prickly heat, etc.(2) Dilution powder: Dilution powder is a diluted powder made by adding a certain amount of filler to a small dose of highly toxic drugs for the next step of preparation. The dilution factor is determined by the dosage: a dosage of 0.1-0.01g can be made into a 10-fold powder (i.e., a powder mixed uniformly with 9 parts diluent and 1 part drug), 0.01-0.001g can be made into a 100-fold powder, and below 0.001g should be made into a 1000-fold powder.The highlighted parts are key points; the rest is for your understanding!That’s all for today; we will continue tomorrow!Welcome to follow Little Pharmacy, let’s explore pharmacy together!


